Hi Everyone!! This article will share Class 10 Carbon and Its Compounds MCQ – Term 2.
In my previous posts, I have shared questions and answers of Heat and Its Effect and Light Shadows and Reflection so, you can check these posts as well.
Class 10 Carbon and Its Compounds MCQ – Term 2
1. Carbon exists in the atmosphere in the form of
(a) carbon monoxide only
(b) carbon dioxide only
(c) carbon monoxide and carbon dioxide in traces
(d) coal
2. Food, clothes, medicines, books, etc. are all based on
(a) oxygen
(b) carbon
(c) nitrogen
(d) none of these
3. The amount of carbon present in the earth crust as minerals is
(a) 0.02%
(b) 0.03%
(c) 0.04%
(d) 0.05%
4. The property of self-linking among identical atoms to form long chain compounds is known as
(a) Catenation
(b) Polymerisation
(c) Isomerisation
(d) Halogenation
5. Carbon attain stability by _______ its valence electrons.
(a) Transferring
(b) Gaining
(c) Sharing
(d) Donating
6. Which is not correct regarding isomers?
(a) They may have same or different chemical properties.
(b) They have different physical properties.
(c) They have same molecular mass.
(d) They have same structural formula.
7. Which of the following statements are usually correct for carbon compounds? These
(i) are poor conductors of electricity.
(ii) are good conductors of electricity.
(iii) don’t have strong forces of attraction between their molecules.
(iv) have strong forces of attraction between their molecules.
(a) ii & iv
(b) i & iv
(c) ii & iii
(d) i & iii
8. Identify the unsaturated compounds from the following:
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) i & ii
(b) ii & iv
(c) iii & iv
(d) ii & iii
9. How many double bonds are there in a saturated hydrocarbon?
(a) Zero
(b) One
(c) Two
(d) Three
10. The isomeric pair is
(a) Ethane and propane
(b) Butane and 2-methylpropane
(c) Ethane and ethene
(d)Propane and butane
Class 10 Carbon and Its Compounds MCQ – Term 2
11. Which among the following has the longest chain?
(a) 2,2-dimethylbutane
(b) 2-methylpentane
(c) Iso-pentane
(d) neopentane
12. The electronic configuration of an element is found to be 2, 4. How many bonds can one carbon atom form in a compound?
(a) 1
(b) 2
(c) 4
(d) 6
13. Which of the following is not considered as crystalline allotrope of carbon?
(a) Fullerene
(b) Diamond
(c) Graphite
(d) Coal
14. C3H8 belongs to the homologous series of
(a) Alkanes
(b) Alkenes
(c) Alkynes
(d) Cycloalkanes
15. Buckminsterfullerene is an allotropic form of
(a) Tin
(b) Carbon
(c) Sulphur
(d) Phosphorus
16. In which of the given compounds -OH is the functional group?
(a) Butanal
(b) Butanol
(c) Butanone
(d) Butanoic
17. Who prepared urea the first time by heating ammonium cyanate?
(a) Wohler
(b) Walter
(c) Walton
(d) Lavosier
18. Structural formula of ethyne is
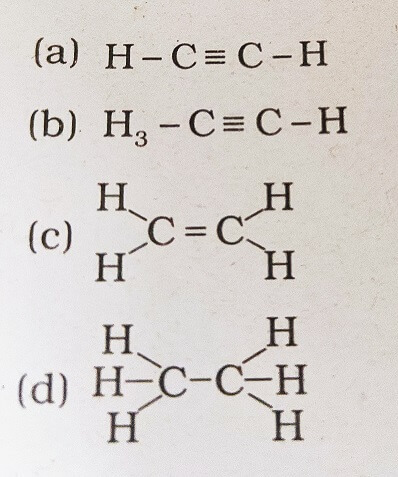
Answer: (a)
19. Combining capacity of various elements depends on the:
(a) Valence electrons
(b) Total number of electrons
(c) Number of neutrons
(d) Both (a) and (b)
20. The number of isomers of pentane is
(a) 2
(b) 3
(c) 4
(d) 5
Class 10 Carbon and Its Compounds MCQ – Term 2
21. Name the functional group present in CH3COCH3.
(a) Carboxylic acid
(b) Alcohol
(c) Aldehyde
(d) Ketone
22. Which of the following belongs to homologous series of alkynes?
C3H4, C6H6, C2H4, C2H6.
(a) C2H4
(b) C6H6
(c) C3H4
(d) C2H6
23. Which of the following doesn’t belong to the same homologous series?
(a) CH4
(b) C2H6
(c) C3H8
(d) C4H8
24. The portion left on dropping a hydrogen atom from an alkane is called
(a) alkenyl group
(b) functional group
(c) alkyl group
(d) phenyl group
25. Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
(a) Argon
(b) Neon
(c) Helium
(d) Krypton
26. How many electrons are there in the outermost orbit of carbon?
(a) One
(b) Two
(c) Three
(d) Four
27. Hydrocarbons are mainly composed of which of these?
(a) Hydrogen, oxygen and carbon
(b) Hydrogen, carbon and nitrogen
(c) Hydrogen
(d) Hydrogen and carbon
28. Successive members of a homologous series vary by how many atomic mass unit?
(a) One
(b) Thirteen
(c) Fourteen
(d) Sixteen
29. Which of these is true for most of the organic compounds?
(a) High melting and boiling points
(b) Low melting and boiling points
(c) High melting point but low boiling point
(d) Low melting point but high boiling point
30. Major constituent of LPG is________
(a) Ethene
(b) Propane
(c) Butane
(d) Pentane
Class 10 Carbon and Its Compounds MCQ – Term 2
31. The first member of alkyne homologous series is:
(a) Methane
(b) Ethene
(c) Ethyne
(d) Propyne
32. Pentane has the molecular formula C5H12 has
(a) 10 covalent bonds
(b) 12 covalent bonds
(c) 14 covalent bonds
(d) 16 covalent bonds
33. Which of the following hydrocarbons has alternate carbon to carbon single and double bonds arranged in the form of a ring?
(a) Hexene
(b) Benzene
(c) Cyclohexane
(d) Butene
34. Which of the following is the correct representation of electron dot structure of nitrogen?
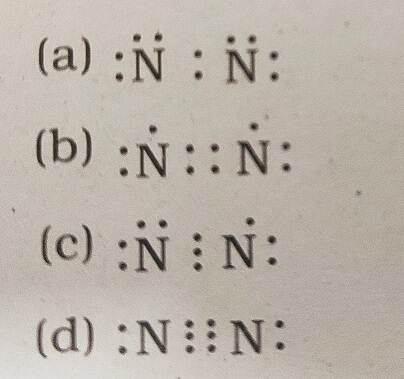
Answer: (d)
35. Which of the following is not a straight chain hydrocarbon?
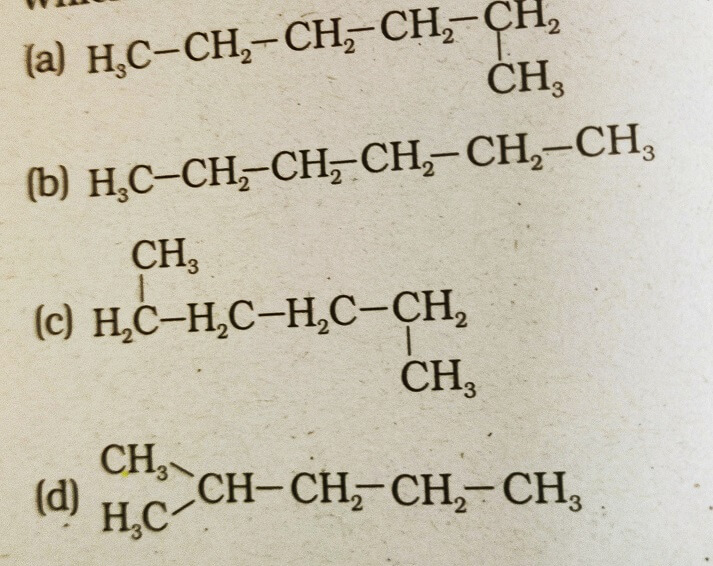
Answer: (d)
36. The heteroatoms present in
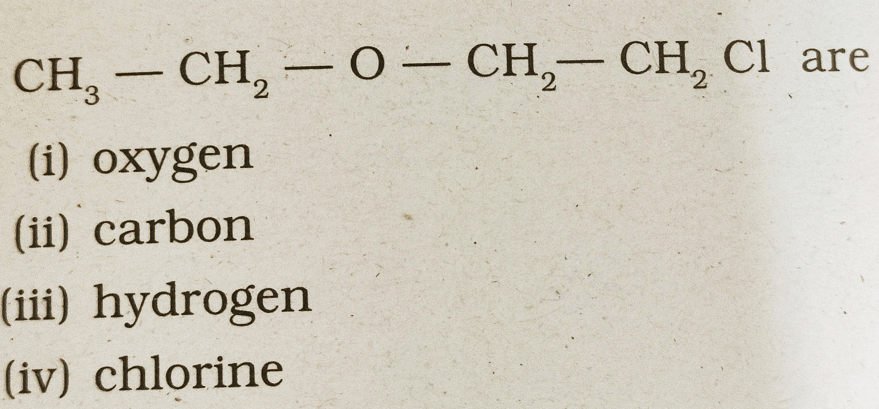
(a) i & ii
(b) ii & iii
(c) iii & iv
(d) i & iv
37. The correct electron dot structure of a water molecule
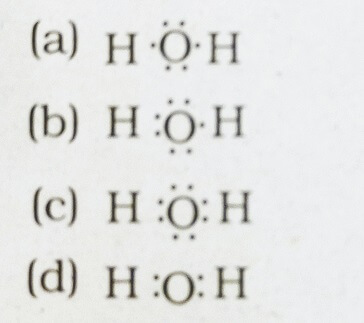
Answer: (c)
38. Which of the following are correct structural isomers of butane?
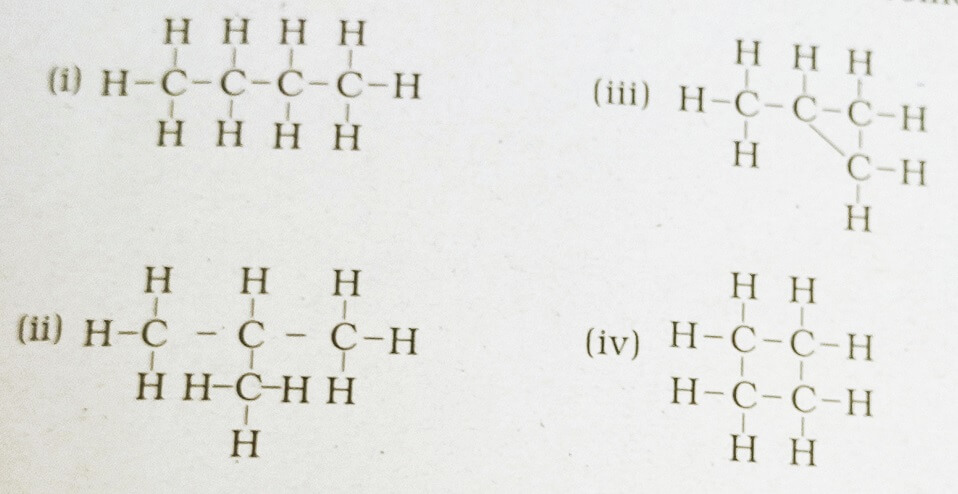
(a) i & iii
(b) ii & iv
(c) i & ii
(d) iii & iv
39. A molecule of ammonia (NH3) has
(a) only single bonds
(b) only double bonds
(c) only triple bonds
(d) two double bonds and one single bond.
40. Structural formula of benzene
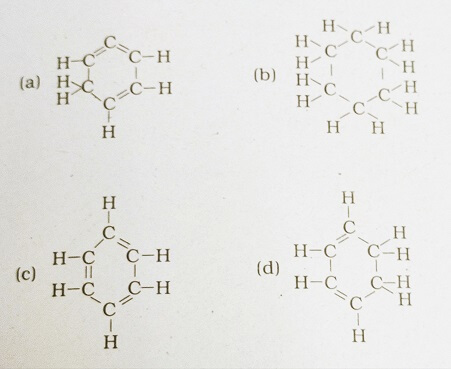
Answer: (c)
Class 10 Carbon and Its Compounds MCQ – Term 2
41. The correct structural formula of butanoic acid
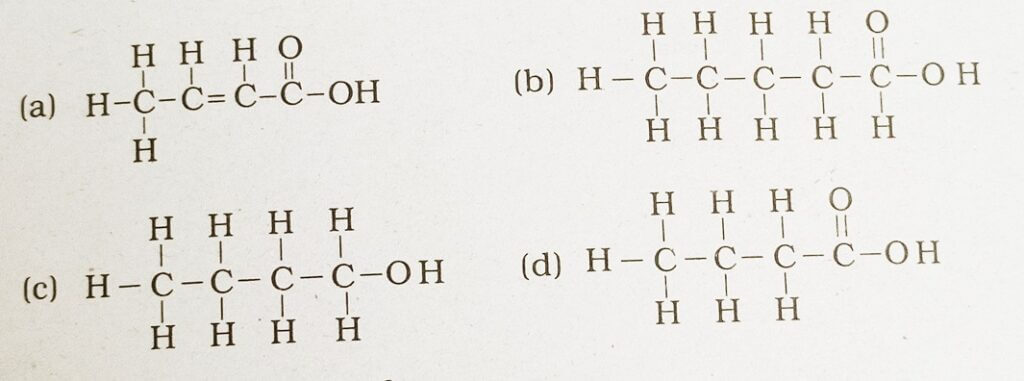
Answer: (d)
42. Butane has the molecular formula C4H10 has
(a) 7 covalent bonds
(b) 10 covalent bonds
(c) 12 covalent bonds
(d) 13 covalent bonds
43. An unsaturated hydrocarbon having a triple bond has 18 hydrogen atoms in its molecule. The number of carbon atoms in this molecule is:
(a) 8
(b) 10
(c) 18
(d) 20
44. The functional group that never occurs as a terminal group is
(a) Carboxylic group
(b) Ketonic group
(c) Aldehydic group
(d) Alcoholic group
45. How many carbon atoms are there in a molecule of 2,2-dimethylpropane?
(a) 3
(b) 4
(c) 5
(d) 6
46. The molecular formula of the third member of the homologous series of ketone is:
(a) C4H8O
(b) C3H6O
(c) C5H10O
(d) C6H12O
47. Alcohols may be represented by the general formula:
(a) CnH2n+2 –OH
(b) CnH2n+1 –OH
(c) CnH2n –OH
(d) CnH2n-1 –OH
48. Alkynes may be represented by the general formula:
(a) CnH2n+2
(b) CnH2n+1
(c) CnH2n-1
(d) CnH2n-2
49. The number of electron pairs shared by the two carbon atoms which are bonded by a triple bond are:
(a) One pair
(b) Two pairs
(c) Three pairs
(d) Six pairs
50. Name the characteristic features of carbon which when put together give rise to a number of carbon compounds:
(a) Catenation
(b) Tetravalency of carbon atoms
(c) Both (a) & (b)
(d) None of these
Class 10 Carbon and Its Compounds MCQ – Term 2
51. In diamond, each carbon atom is bonded to four other carbon atoms to form
(a) a rigid three-dimensional structure
(b) a hexagonal array
(c) a structure of a ring
(d) a structure in the shape of a football
52. Which allotrope of carbon is in the form of the geodesic globe?
(a) Diamond
(b) Carbon nanotube
(c) Fullerene
(d) Graphite
53. How many carbon atoms are present in one molecule of ethanoic acid?
(a) One
(b) Two
(c)Three
(d) Four
54. Functional group – CHO is present in which of the given?
(a) Alcohol
(b) Carboxylic acid
(c) Aldehyde
(d) Ketone
55. Which of the given is an odd compound?
(a) Ethane
(b) Ethene
(c) Propene
(d) Acetylene
56. A hydrocarbon should have a minimum of how many carbon atoms to show isomerism?
(a) Three
(b) Four
(c) Five
(d) Six
57. Graphite structure is formed by ______ array:
(a) Tetragonal
(b) Pentagonal
(c) Hexagonal
(d) None of the above
58. Which among the following properties does not comply with graphite?
(a) High melting point close to that of diamond.
(b) Presence of hexagonal rings of carbon.
(c) Electrical conductivity comparable to metals.
(d) Layer tightly held by strong forces of attraction.
59. How many number of carbon atoms are joined in a spherical molecule of buckminsterfullerene?
(a) 30
(b) 60
(c) 70
(d) 80
60. Identify the carbon compound in which carbon doesn’t exhibit the property of catenation as well as multiple bond formation?
(a) Benzene
(b) Propene
(c) Methane
(d) Acetone
So, these were Class 10 Carbon and Its Compounds MCQ – Term 2.